What is HSCT?
Updated 7/14/16
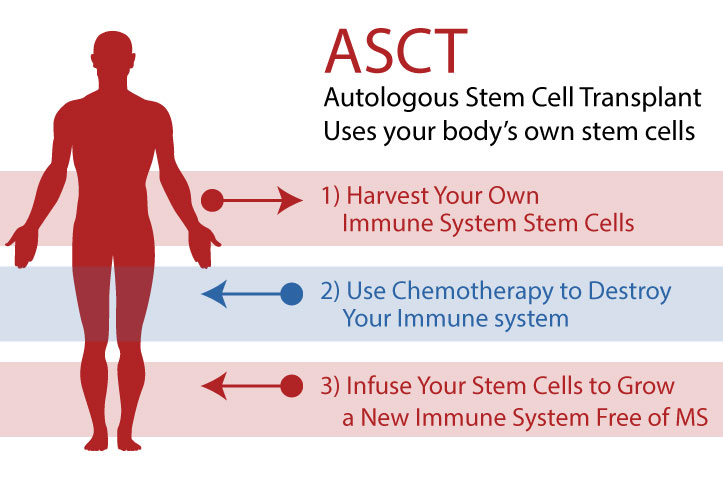
Analogous Hematopoietic Stem Cell Transplant, sometimes abbreviated as ASCT or HSCT, is a process by which your own (analogous) hematopoietic stem cells (i.e. immune system stem cells), grown in your own bone marrow, are harvested from your body (usually through your blood), then given back to you after chemotherapy (which wipes out your existing immune system) to rebuild a new immune system (without memory of your autoimmune disease) from scratch.
This is in effect a reboot of the immune system.
There are many types of stem cells in the body, each of which can grow into particular types of new cells. Hematopoietic stem cells grow into new immune cells, and they are abundant and easy to capture. Furthermore, a mobilizing drug (i.e. filgrastim) can be used to encourage the bone marrow to generate HSCs by the millions.
HSCT (aka ASCT) for autoimmune disease (i.e. MS) is not yet FDA approved in the USA, and therefore some Western Medicine neurologists have little or no idea what the treatment is, what it does, or how safe it is. In the last couple of years, they've finally started catching-on as more patients return from foreign countries with incredible results.
The process was first developed in the 1950s as a Nobel Prize-winning procedure to treat certain types of cancer. In the late 1960s, it was further used to treat lymphoma. In the mid-1990s, a version of the method was pioneered by Dr. Richard Burt and Dr. Shimon Slavin to target autoimmune diseases, and by the mid-2000s, the procedure had been modified from myeloablative to non-myeloablative, significantly decreasing mortality rates.
But isn’t myeloablative more effective?
“No, it is not, as far as MS and other autoimmune illnesses are concerned,” Dr. Fedorenko said.
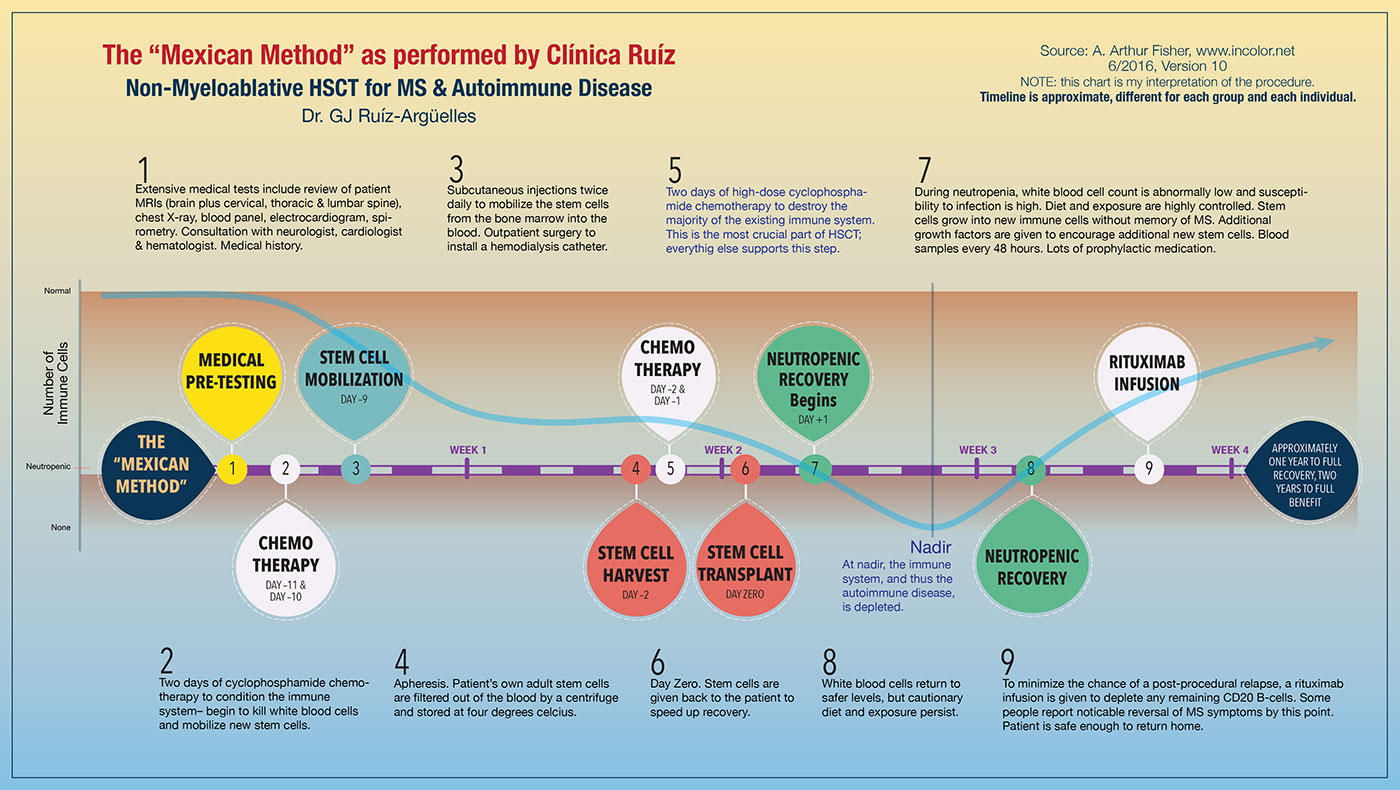
“Cancer is a fatal illness and so our goal is to save the patient’s life. We have to treat both red and white cells. But MS is not fatal and so our goal is different. Now we are seeking to halt the disease and give the patient an improved quality of life. To do this, we only need to treat the lymphoid cells, not the red cells that carry oxygen. And that, in simple terms, is the difference between myeloablative and non-myeloablative HSCT.[1]
In the myeloablative procedure, all the body's immune cells are completely destroyed, including those in the blood and bone marrow. In the non-myeloablative procedure, only adult immune cells that exist in the blood are destroyed, leaving the bone marrow and existing stem cells intact. The non-myeloablative procedure is both safer and provides for a faster recovery. In the early 2000s, mortality rates dropped nominally from < 5% to < 0.5%, a tenfold improvement, as the process preference changed to non-myeloablative. Based on my calculations, the 2010s showed mortality rates further dropping nominally to less than 0.2% as the process was further refined and pre-treatment testing was enhanced.
As of this writing (7/2016), there have been over 500,000 HSCTs performed since its inception, and currently, some 50,000 HSCT procedures are completed each year (mostly for cancer)[2]. Approximately 1,500 - 2,500 HSCTs have been performed for MS and autoimmune disease with a cumulative mortality rate of < 1%[3]. But since the procedure has been refined over the years, recent figures show that it's become even safer. As of June 2016, between Moscow and Mexico alone, over 660 patients have completed HSCT[4] for MS with a near-zero mortality rate[5]. I did read about one woman from Brisbane with late-stage, chronic inflammatory demyelinating polyneuropathy who died from heart failure after the procedure in Moscow, but it's unclear if HSCT was the direct cause. Another died unexpectedly shortly after a severe reaction to chemo done in Israel. We do know that patients with co-morbidities are not good candidates for safe HSCT, and your body must be strong enough to withstand high-dose chemo (usually cyclophosphamide). Otherwise, the safety is almost on par with some of the dangerous DMT's (especially if you're JC positive) we might otherwise be taking.
Today, survival rate (of the procedure--do not confuse this term with mortality rate) at five years, drug-free and relapse free, is generally better than 80%. Few patients experience a relapse or continuing progression. About half of patients experience a significant reversal of pre-treatment disability. In Dr. Burt's Stage 3, International Clinical Trial, the average quality of life increased over 33% and EDDS scores decreased by well over 1 full point.[6]
The fact is that HSCT is currently the only option available to address autoimmune diseases which has a possibility of significantly increasing quality of life, in addition to stoping the disease progress! And it's the only one that can do so without the need for further DMTs[7].
HSCT is based upon the idea that:
- a patient can generate a large number of hematopoietic stem cells (HSCs), which can be harvested and stored for future use.
- the patient's immune system can be effectively destroyed with chemotherapy without harming the patient's other bodily functions.
- a patient can survive without an immune system for a short period of time if kept in a clean environment and with the help of antiviral medication.
- the patient's harvested HSCs can be transplanted back into the patient, where they will effectively assist the patient's bone marrow to regrow a new immune system.
- the patient's new immune system will have no "memory" acquired by the old immune system, including a memory of autoimmune
1. HSCT: Denis Fedorenko, a Family Man Who ‘Instills Confidence’. 2016. http://multiplesclerosisnewstoday.com/blog/2016/07/08/hsct-dr-fedorenko-the-family-man-treating-hundreds-of-ms-patients/ (link now broken, see:) https://hsct-russia.com/about-us
2. George Goss, themscure.blogspot.com
3. ibid.
4. Over 550 cases completed in Moscow: source, Alex Greene Interview with Dr. Federenco, May 11, 2016. 114 cases treated at Clínica Ruiz as of 6/17/16: source, Dr. Ruíz-Arqüelles.
5. ibid.
6. Dr. Richard Burt presentation, Cellular Horizons 3rd International Conference, April 28, 2016
7. ibid.
Important Timeframes
- My Stem Cell Transplant Date: June 5th, 2016
- Treatment duration: 4 weeks
- Typical onset of disability reversal: +9 months
- Typical complete recovery from procedure: +1 to +2 years
- Typical maximum reversal of disability: +2 years
Disclaimer
I am not a doctor. I am a scientist (engineer) who has had MS since March 2013.
Explanation Video
This video explains HSCT in the words of one of the procedure's primary pioneers. If you do nothing else on this website, watch this presentation.
This video is a presentation made by Dr. Richard Burt, a leading pioneer of the HSCT for the autoimmune disease process. This is the single, most comprehensive description of what MS is and why HSCT is so important:
Note that I applied to Dr. Burt's clinical trial and was rejected as I hear most everyone is. He seems to want only people who get really sick, really fast but haven't been sick for terribly long.
The website blogs are separated into two sections: the Treatment Blog and the Recovery Blog. Day Zero is when I received my stem cells back.
- Homeward Bound (Day +12)
- Treatment Complete (Day +11)
- Final leg of the procedure (Day +10)
- Healing fast! (Day +8)
- Neutropenia (Day +6)
- Jumped the gun (Day +3)
- Immune System Gone (Day +2)
- DAY ZERO - Got My Stem Cells Back!
- One million stem cells per kilo (Day -2)
- The “Mexican Method” (Day -4)
- Portal to the heart (Day -5)
- Waiting out mobilization, (Day -8)
- Done with first rounds of chemo (Day -9)
- A Rough Night (Day -10)
- Starting chemo now! (day -11)
- An Easy Day (day -12)
- First Major Snafu - The Flight Agent Chronicles
- We’re off
- Countdown to vacate
- Everything's Confirmed
- AHSCT and Progressive MS. Conflicting Studies (Year 6.4) - UPDATED
- Check-In (Year 5.2)
- No New Activity on MRIs (Year 3.6)
- Bloodwork Back To Normal (Year 3.3)
- Why Neurologists in the US Don't Recommend HSCT (Month +35.8)
- Better Pain Management (Month +33.6)
- Final Re-Vaccinations & The Medical Cannabis Primer (Month +32.9)
- "Sustained Decrease in Disability" (+30.6 months)
- No Progression Seen on MRI (Month +29.7)
- Cruising Along (Month +29)
- Nerve Decompression Surgery (Month +21.8)
- Looks Like It Worked (Month +17.2)
- Further Pain Improvements (Month +15)
- Significantly Lower Pain (Month +14.4)
- More Thoughts About MMJ, Meds & Pain (Month +13)
- +1 Years and Counting! (Month +12.6)
- Better & Better (Month +11.5)
- Major Pain Improvements. Finally. (Month +10.6)
- DIMS, SIMS and the Medicine Cabinet in the Brain (Month +9.6)
- Sliding Sideways ( Month +9.2)
- Pain, Pain, Go Away! (Month +8)
- Doctor: MS in Remission! (Month +6.75)
- Neuroplasticity (Month +6.1)
- Hopefully, My Last MRIs, Forever (Month +6)
- Chemobrain (Month +5.2)
- Kickin' and Screaming (Month +5)
- Month +3.7 Progress Report
- Fresh Foods Welcome! (Month +3.2)
- Thoughts About Pain (Month +3)
- Making Good Progress (Week +12)
- First Followup Rituximab Infusion scheduled (Week +9.5)
- Walking Towards Improvement (Day +54)
- Neutrophils Recovering Appropriately (Day +39)
- Less Pain Lately. Horray! (Day +25)
- The Highs & Lows of Recovery (Day +22)